In an attempt to source cheaper healthcare alternatives, some consumers have turned to risky behaviors, including medicating themselves with animal drugs. One type of product sold online that has high risk for this kind of misuse is fish antibiotics. Given that fish and humans share many of the same medicines (e.g., amoxicillin, penicillin, tetracycline, and more), many consumers believe these piscine pills are an economical equivalent to more costly prescription drugs. However, the Food and Drug Administration (FDA) has warned that these products have not been evaluated for safety and effectiveness - making it illegal to market them and problematic for payment service providers who process payments for these merchants.
Even though merchants may intend for the antibiotics they sell to treat fish, reviews on websites often allude to or explicitly state that customers are purchasing them for human use.
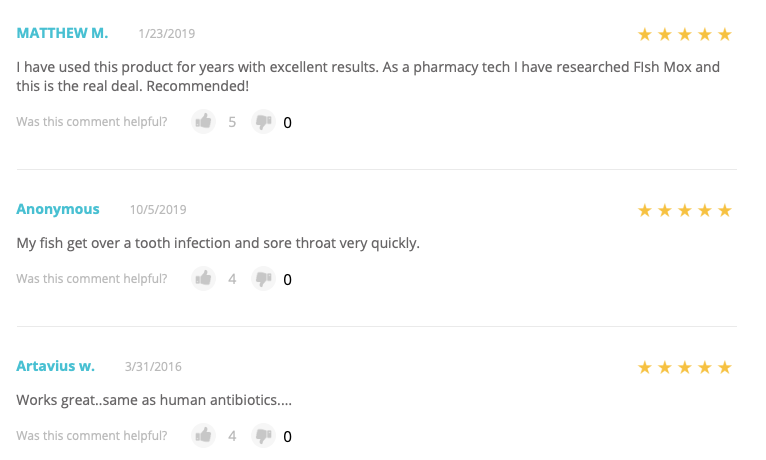
Online reviews of fish antibiotics on a popular website selling pet products
According to what customers state in reviews, fish antibiotics have been used to treat a variety of infections and diseases, including tooth abcesses, urinary tract infections, bronchitis, Lyme disease, and more. Consumers, who may be unable or unwilling to visit a doctor for treatment, may see fish antibiotics as a simpler, cheaper alternative to the prescription drugs and doctors' copays.
However, while human and animal medications share many similarities, the fish antibiotics sold online and in pet stores are for "minor species," and therefore may escape FDA scrutiny. That means these drugs may sidestep what can be important quality-control measures. Though these products generally have not been approved, conditionally approved, or indexed by the FDA to permit them "legal marketing status," many manufacturers continue to bypass these requirements and offer their products on the shelves.
"These unapproved products may not meet the agency's strict standards for purity and potency," the FDA said in a statement on its website. "And, we don't know how the products have been handled and stored because they haven't been controlled by a veterinarian or pharmacist."
Given the lack of FDA oversight, these products may be especially risky for humans. While some fish antibiotics are marketed as being "pharmaceutical grade," this designation, absent of federal inspection, does nothing to guarantee the purity, handling, or efficacy of the products.

Examples of animal products sold online that contain amoxicillin or erythromycin
Furthermore, self diagnosis and self treatment can be dangerous even if the drugs are of acceptable quality. According to Wilson E. Gwin, director of the Purdue Veterinary Teaching Hospital Pharmacy, it's crucial to match the proper antibiotic to the particular illness. "Let's say the antibiotic is correct, that capsule contains the right amount of medicine, and it's a good quality medication and its able to be absorbed into the system," Gwin said in an interview with Smithsonian Magazine. "We don't really know if that's the right drug for what the person is trying to treat. If it's the wrong drug, they can do themselves even more harm."
Payment service providers should be cautious of any merchants offering fish medication with prescription-only ingredients, as there are limited numbers of fish medicines currently approved by the FDA. You can search the animal drug database on the FDA's website for approved animal medications. Most important, when reviewing a merchant's website, payment service providers should view any marketing indicating that animal drugs are effective for human use as fishy.




