Many cultures associate fair complexions with beauty, prosperity, and better socioeconomic status. As a result, skin lightening products, which typically come in the forms of creams, lotions, and soaps, are popular throughout the world with mostly female consumers. A simple search for “skin lightening cream” yields thousands of results for cosmetic products that can be purchased with just one click on various e-market platforms.
What many people do not know is although lightening products are often marketed as being made from natural ingredients, that isn’t always the case, and it doesn't guarantee they are approved or safe. Regulatory authorities often conduct testing on these products and find them to contain dangerous ingredients, such as mercury, hydroquinone, and clobetasol propionate. These ingredients pose a risk of serious health effects.
Mercury
The US Food and Drug Administration (FDA) prohibits mercury in drugs and cosmetics, except under very specific conditions. Exposure to mercury can cause permanent damage to the brain and kidney, skin rashes, skin discoloration and scarring, and a reduction in the skin’s resistance to bacterial and fungal infections. Furthermore, the FDA states that consumers using products containing mercury can expose family members through skin contact or mercury vapor, putting them at risk. Sellers of mercury-laden skin lightening creams in the US may be subject to enforcement action, including seizure of products, injunctions, and criminal prosecution.
Due to the widely known safety risks associated with mercury, many manufacturers do not disclose the mercury content in their products, which can lead to severe outcomes. For example, the California Department of Public Health has documented a number of cases of mercury poisoning resulting from adulterated skin-lightening creams, some of which were found to contain up to 21 percent mercury. These cases included a 20-month-old child exposed to mercury through a skin cream utilized by its mother; contact tracing revealed that up to 40 individuals in six households were exposed.
Hydroquinone
Many skin lightening products also often contain high concentrations of hydroquinone. Historically, drug products containing no more than 2% hydroquinone were permitted to be sold over the counter in the United States. OTC monograph reform, included in the Coronavirus Aid, Relief, and Economic Security Act (CARES Act) of 2020 eliminated the non-final skin bleaching monograph which had been in place since 1982. FDA approval is now required for all hydroquinone products before they may be sold in the US. Currently, there are only a few FDA-approved drug products containing hydroquinone, and they are only available with a prescription.
Hydroquinone is also banned in cosmetics in multiple jurisdictions, including the European Union (EU), Ghana, Rwanda, and Kenya. Nonetheless, it is used in medicines for pigmentary disorders. Lower strength hydroquinone products can be found sold over the counter; however, higher concentrations generally require a prescription from a physician. Regulators warn that the use of these products can put users at risk of leukoderma (depigmentation), ochronosis (hyperpigmentation), allergic contact dermatitis, and possibly cancer.
Clobetasol Propionate
Clobetasol propionate is a topical corticosteroid that’s commonly used to treat inflammation and irritation on the skin. Products containing clobetasol generally require a prescription and are meant to be used short term only. According to authorities, using clobetasol for an extended time can cause the skin to become thinner and create stretch marks. Cushing's syndrome has also been reported as a result of prolonged use of topical clobetasol products. Furthermore, a large amount of clobetasol passing through the skin can shut down the adrenal glands, which can lead to nausea, vomiting, fever, low blood pressure, heart attack, and even death because the body cannot appropriately respond to stress or illness.
As shown in the screenshot below, a skin lightening product marketed on an e-commerce platform appears to be natural because the packaging states that it is made with lycopene, avocado, and aloe vera.
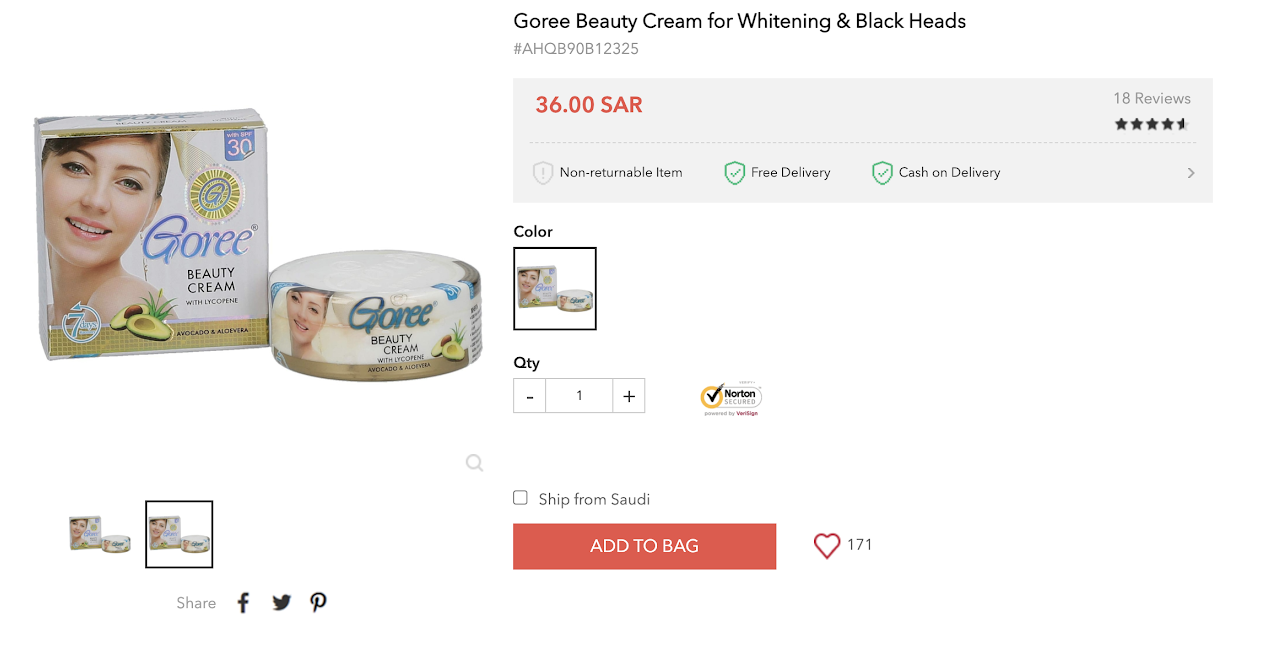
A merchant on an e-commerce website markets Goree skin lightening cream.
However, the FDA has called out this product for containing high levels of mercury, as shown below.
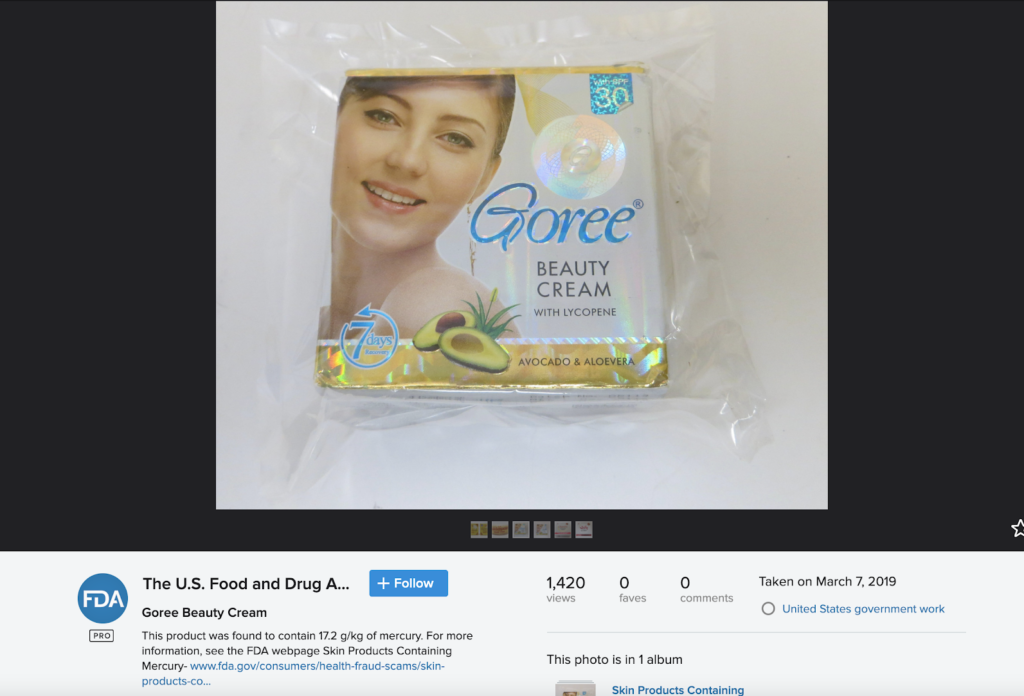
The FDA found that Goree skin lightening cream contained mercury.
Skin lightening products not only endanger the public’s health, but also pose high risk to the e-commerce platforms that host them. Violative products can put online marketplaces at risk of reputational damage and even action by regulatory authorities. Demand for these products is unlikely to subside, but e-commerce platforms can diligently monitor for and remove these products to help protect themselves and consumers. LegitScript monitors online marketplaces for dangerous healthcare products, including adulterated skin lightening cosmetics, to protect the public from dangerous goods and help e-commerce marketplaces stay in compliance.
Download our full cosmetics guide for more information related to risks that come with cosmetic products.





