Ear candling, also referred to as "coning," is a popular practice believed to draw wax and other impurities out of the ear. Despite their widespread availability, these products are unapproved medical devices that can leave both consumers and payment service providers burned. Read on to find out what these products are and the regulatory framework around them.
What are ear candles?
Ear candles are hollow cones that have been soaked in beeswax or paraffin and hardened. During ear candling, a person will typically lie flat on one side while the cone is inserted into their ear. Once positioned, the top of the cone is set on fire, burning for a few minutes. Proponents believe that the heat from the cone will create a suction for wax and debris to be removed from the ear canal. In addition to wax removal, some ear candles have claimed to help with a laundry list of medical issues, including earaches, sinus pain, hearing loss, and even cancer. In addition to the lack of evidence around ear candles' efficacy to treat any of these conditions, use of the products has resulted in multiple injuries, which is why regulatory agencies are voicing their concerns.

An online merchant makes claims that ear candles can treat a variety of ailments, including infections, hearing loss, allergies, tinnitus, vertigo, and more.
What do regulators say about ear candles?
The United States Food and Drug Administration (FDA) and the Department of Health Canada have warned the public of serious injury related to ear candling, as they state there is no valid scientific evidence for any medical benefit from their use. The FDA and Health Canada report on multiple cases of injury caused from ear candles, and both administrations list these common risks:
- Objects catching fire;
- Burns to the face or ear canal;
- Ears plugged by wax;
- Punctured eardrum;
- Temporary hearing loss.
One FDA warning reports of a patient who required surgery due to serious damage caused by an ear candle. Some ear candles even claim to help children and babies, causing great concern, as the risks listed above have the potential to be even greater in small children. Ear candles are considered to be unapproved and misbranded medical devices in the US and Canada. The FDA has issued multiple warning letters to manufacturers who were marketing their ear candles with such impermissible claims.
Where are ear candles sold?
Although there are multiple warnings and risks associated with ear candling, ear candles are sold frequently on the internet and in major retailers. Large companies, such as Target and Walmart, as well as independent healthcare stores, offer them within their catalogs. It is also common to see ear candling services offered in local healing centers and massage offices. Although ear candling may be administered by a professional in the office, the risks are still present. Ear candles have become widely popular within the healthcare realm; however, it is important for consumers to understand the dangers and risks they pose. Next time you feel a need for cleaning out your ears, you may want to consult a doctor who will use approved methods, rather than ear candling at home.
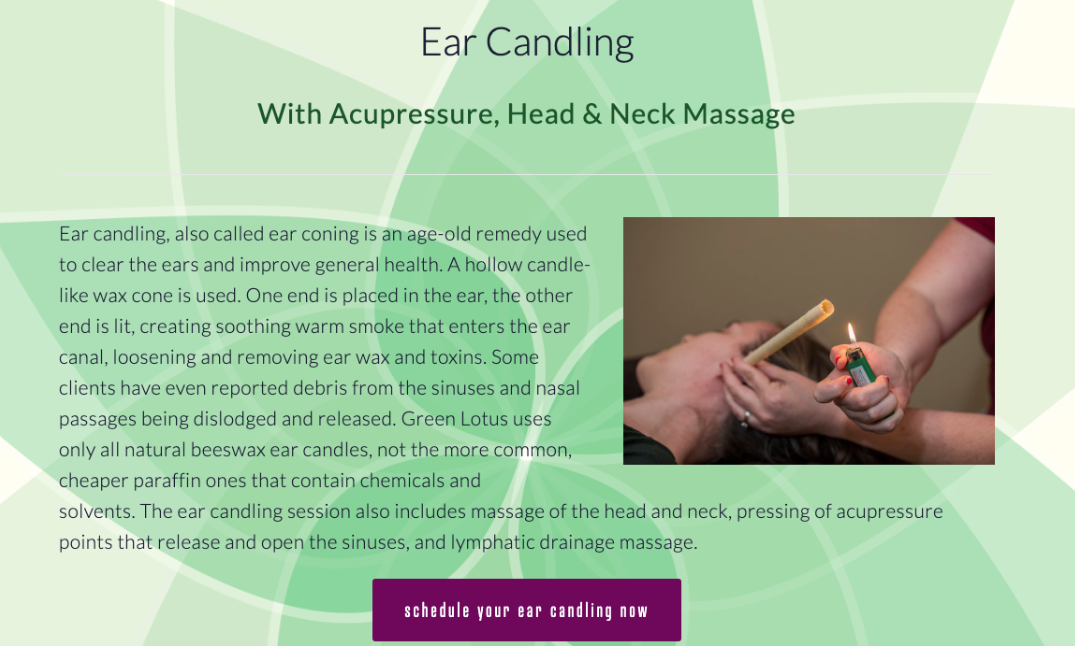
A merchant offers ear candle services in conjunction with acupressure and massage.
In addition to ear candles, LegitScript monitors the internet and payment ecosystems for other unapproved medical devices and problematic products, as well as dozens of other high-risk categories. Our merchant monitoring and platform monitoring solutions combine big data with a team of human experts to shine a bright light on merchant activity, so you can confidently assess merchant risk and take action before it results in fines or regulatory intervention. What to learn more about how LegitScript can help you reduce risk? Contact us to learn more.




