Before 2020, when the pandemic limited access to in-person healthcare, telehealth was growing only incrementally and some well-meaning laws made it difficult to access certain medications. Now, newly proposed federal rules take into account how society has changed in a post-COVID world, and how it’s impacted the prescribing of controlled substances online. Read more to understand what new rules the DEA is proposing, and their potential to permanently change the telemedicine landscape.
Telehealth Became More Popular During the Global Pandemic
In 1925, a popular science magazine published an article ideating a device that allowed for video examination — and the concept of telehealth was born.
The path to widespread use of video telemedicine has been building slowly over decades. Telehealth’s compound annual growth rate (CAGR) — the rate of return required for an investment to grow comparatively from beginning to end — was 13% in 2018.
Enter the global pandemic.
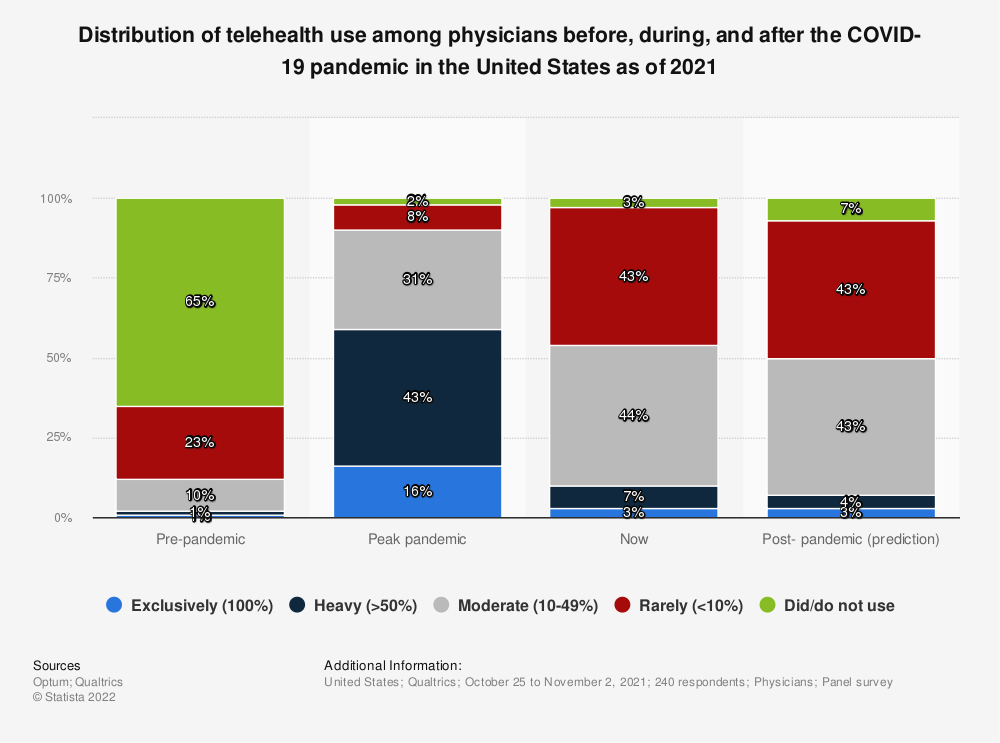
By 2021, during the pandemic’s peak, 98% of physicians were using some form of telehealth (as shown in the chart below). Now, the latest projections indicate telemedicine CAGR will rise to 25.7% between 2023-2032.
One of the major sticking points during the pandemic? Patients needing controlled substances couldn’t get access to them through telemedicine. A law that passed more than a decade earlier — the Ryan Haight Act — made that extremely difficult.
The Ryan Haight Online Pharmacy Consumer Protection Act of 2008 Included These Two Significant Rules
The advent of cheap and powerful synthetic opioids has helped to fuel an increasing number of overdose-related deaths since 1999. The Centers for Disease Control (CDC) named it an epidemic as the death toll mounted — a toll that, in 2001, included an 18-year-old named Ryan Haight. His death prompted the 2008 Ryan Haight Online Pharmacy Consumer Protection Act, which required a prescribing practitioner to have conducted at least one in-person medical evaluation of the patient before prescribing controlled substances.
The amendments the Ryan Haight act made to the Controlled Substances Act included two primary rules:
- There had to be at least one in-person medical evaluation before a practitioner could prescribe controlled substances.
- It modified registration requirements for online pharmacies.
In 2020, the pandemic made access to in-person visits more difficult, which is why the Drug Enforcement Administration (DEA) and Substance Abuse and Mental Health Services Administration issued emergency policies that relaxed controls under the Ryan Haight Act and the Controlled Substances Act on prescribing and dispensing controlled substances via telehealth.
After a few years under these relaxed rules, the DEA is proposing new regulations to carry forward after the projected expiration of the COVID-19 public health emergency — new rules that could expand access to certain drugs and impact telemedicine’s projected CAGR again.
These Proposed Regulations Could Impact the Way Patients Receive Healthcare — Forever
The newly proposed federal regulations would allow for the continued prescribing of certain non-narcotic controlled substances via telemedicine without an in-person consultation in limited circumstances.
Under these proposed rules:
- Schedules II-IV controlled substances can be prescribed for 180 days after the expiration of the COVID-19 public health emergency.
- A practitioner may prescribe controlled substances without an in-person visit as long as there is an audio-visual telehealth evaluation. They may also prescribe a controlled substance if a referring provider performed the in-person evaluation.
- Interactive telecommunications would include two-way, real-time audio without a requirement for video if the physician or practitioner is at a “distant site” and the patient isn’t able or does not consent to the use of video technology.
- Enhanced record keeping is now required for both the referring and prescribing practitioners — including an annual review of Prescription Drug Monitoring Program’s (PDMP’s) data.
- First-time prescriptions for Schedule III-V controlled substances can only be for 30 days or fewer. An in-person visit is required before the next prescription; however, a patient can visit with a referring practitioner in-person while on an interactive video link with the prescribing practitioner in lieu of an in-person visit.
- The patient can still receive Schedule II controlled substances if an in-person evaluation is performed by a referring or prescribing provider.
According to the DEA, the new rules would give prescribing physicians the opportunity to ensure patients get the medications they need while still providing adequate gates to controlled substances. These new rules could be what ushers in a new era of telemedicine.
How We Can Help You
Are you a telemedicine provider looking to build trust and demonstrate compliance with patients and business partners? LegitScript’s Healthcare Merchant Certification is recognized by major search engines, e-commerce platforms, and payment service providers. Learn more about how LegitScript certification can help you grow your business with confidence.




