The global cosmetics market is flooded with products that claim to help users achieve a fairer complexion and even skin tone. While many of them may be effective, using these products can come with risks that are sometimes hidden from consumers and the merchants offering them.
Keep reading to understand risks and relevant trends with these products, and how LegitScript’s work mitigates their potential for harm. Then download our High-risk Products Handbook for more useful tips about other problematic products.

July 23, 2025 | by Justin Ridderbos
Skin Lightening Is a Multibillion-Dollar Industry
The appeal of lighter skin is not a new phenomenon. From Victorian-era ideals of pale skin as a symbol of wealth to modern-day beauty standards in parts of Asia, Africa, and Latin America, the desire for a lighter complexion continues to drive demand. A market report from strategyr.com projects that the global market for skin lightening products will top $13 billion by 2030. Despite modern changing views around colorism and representation in media, demand for these products continues to persist.
Demand for Skin Lightening Products Is Global
The drivers of demand for these products are complex and vary from culture to culture; however, trends in Google searches show a consistent interest from customers in purchasing them across the globe. While most relevant search volume appears to be concentrated in the global south, LegitScript analysts frequently identify online marketplaces based in the US, UK, and Canada that cater to diasporas from South Asia, Sub-Saharan Africa, and the Caribbean. Products featured on these websites often advertise skin lighteners marketed as being “natural” or “herbal,” when in fact many of them have been found to be tainted with heavy metals or undeclared pharmaceutical ingredients. In the American market, such tainted products have been a focus of the FDA for some time.
What Is in Skin Lightening Products?
The most prominent problematic ingredients featured in tainted skin lighteners include mercury, hydroquinone, tretinoin, and clobetasol propionate. According to the FDA, exposure to these substances can result in serious, permanent damage to one’s health. Mercury exposure has been linked to permanent brain and kidney damage. Prolonged use of hydroquinone has been associated with hyperpigmentation of the skin, facial swelling, and sensitization resulting in dermatitis. Other prescription-only ingredients, like clobetasol, can lead to severe side effects when misused.
Manufacturers often omit these ingredients from product labels or marketing materials because they are heavily regulated or even forbidden. As a result, consumers may be exposing themselves and their families to unintended health risks under the assumption that the products are safe or natural.
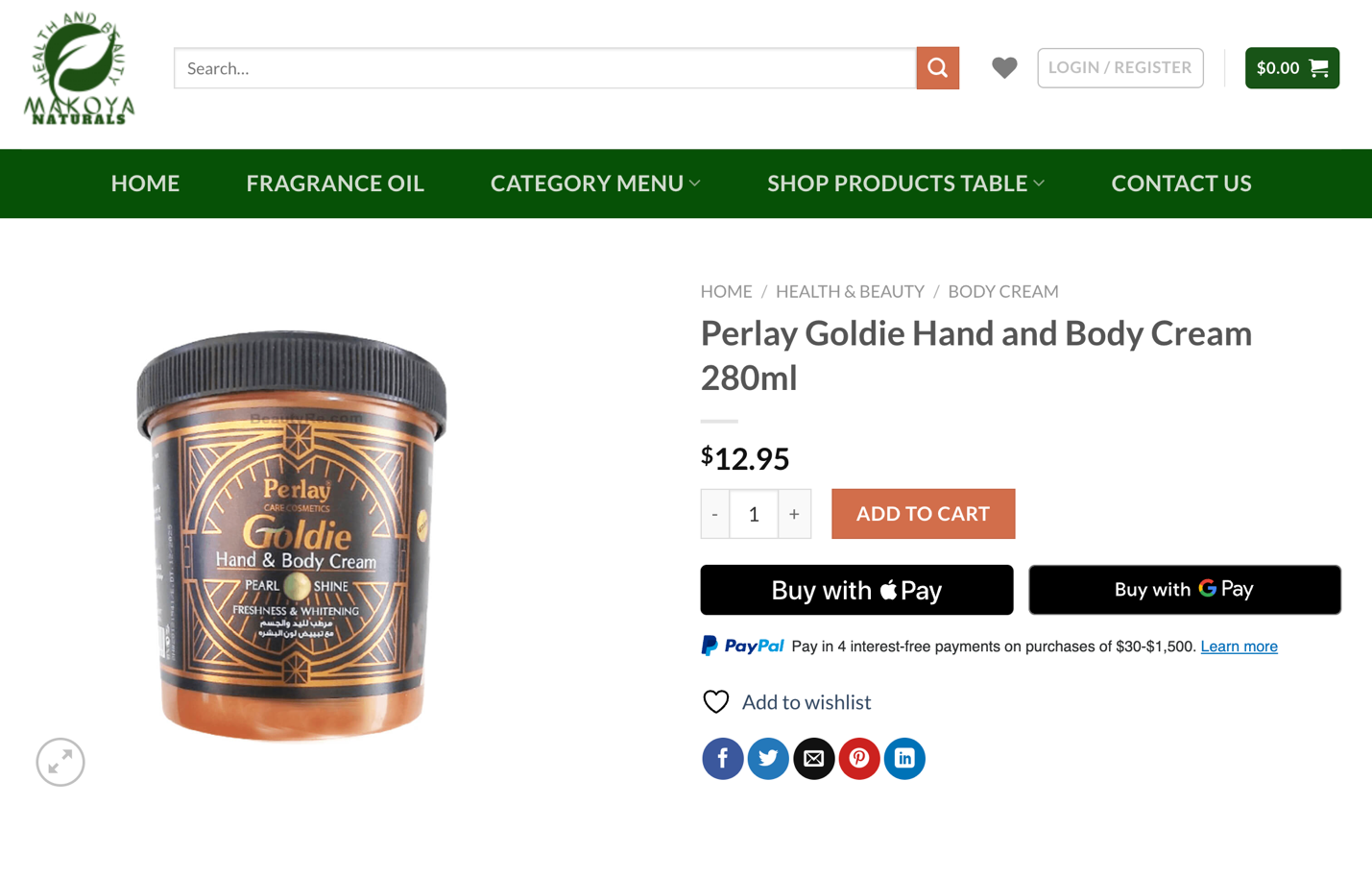
This product, marketed alongside innocuous imported cosmetics and health products, was found to contain dangerous levels of mercury.
What Merchants and Payment Providers Should Know
Regulators and public health agencies continue to take action against tainted cosmetics but enforcement alone isn’t enough. Many products reach consumer markets before being flagged or recalled. That’s why industry monitoring tools play a critical role in identifying risks early.
LegitScript’s Merchant Monitoring services and industry intelligence products help payment providers and e-commerce platforms stay ahead of trends by flagging emerging high-risk products and problematic merchants. Our team of experts track regulatory enforcement, identify banned or hidden ingredients, and provide real-time insights to reduce exposure to risk and keep your customers safe.
Skin lightening cosmetics are just one example of how shifting trends and complex regulations can put consumers and businesses at risk. For more insights on high-risk health and wellness products, download LegitScript’s High-risk Products Handbook. It’s full of practical guidance for identifying dangerous products and managing risk across your portfolio.