Despite continued regulatory action and potentially dangerous health effects, a social media trend promoting Melanotan II — an illicit tanning drug — continues to prevail in the market. LegitScript has observed that in an attempt to skirt account termination and regulatory scrutiny, merchants commonly attempt to disguise their activities with generic packaging and vague marketing, presenting consumer safety risks as well as elevated risk for card brand fines and regulatory scrutiny for payment service providers.
How Does Melanotan II Work and Why is it Trendy?
Melanotan II is a peptide or protein-based drug that acts as a synthetic version of a hormone called α-MSH, which regulates the skin cells responsible for producing pigment. When Melanotan II is injected or used as a nasal spray (its two common forms), it mimics α-MSH, and can cause dramatic skin darkening within days. This melanin-increasing function is its most notorious, but the drug has several other—potentially dangerous—effects and has been reported to cause heart, blood, and eye disorders, among other conditions.
Melanotan’s rapid skin-darkening effect has made it a desirable “shortcut” product for those hoping to achieve the golden, tanned skin that remains a popular symbol of beauty and health. In recent years, Melanotan II has made a particular splash online; a 2022 BBC article calls attention to dozens of influencers promoting the nasal spray to millions of followers on TikTok. LegitScript has noted a correlated increase in merchants marketing nasal tanning products via social media, often with generic, self-branded packaging. Due to the high level of risk associated with Melanotan II, LegitScript is keeping a close watch on the trends and marketing strategies connected with it.
The Dangers of an Illegal and Unregulated Product
Melanotan II was initially developed and touted as a cancer preventative but has since been the subject of bans, warning letters, and even criminal prosecution in multiple jurisdictions. The US Food and Drug Administration (FDA) has not approved the drug for any indication, and regulatory agencies of the United Kingdom, Australia, and New Zealand, among others, have also deemed it illegal to sell.
Users of the nasal spray form of the drug have reported side effects such as headaches, dizziness, panic attacks, nausea, vomiting, facial flushing, unusual moles, and more. It has also been linked to cases of melanoma (3, 4, 6, 8). Aside from the dangers of Melanotan II alone, consumers risk exposure to any number of mystery ingredients. The BBC describes the findings of research chemists at Imperial College London who analyzed 10 tanning kits, “[t]hey would expect to find about 10 ingredients in a licensed medicine,” they write, “but were shocked to discover some of the products contained more than 100 unidentified ingredients.” In attempts to avoid detection and stay in business, sellers of nasal tanning spray often offer little-to-no information about their products’ contents.
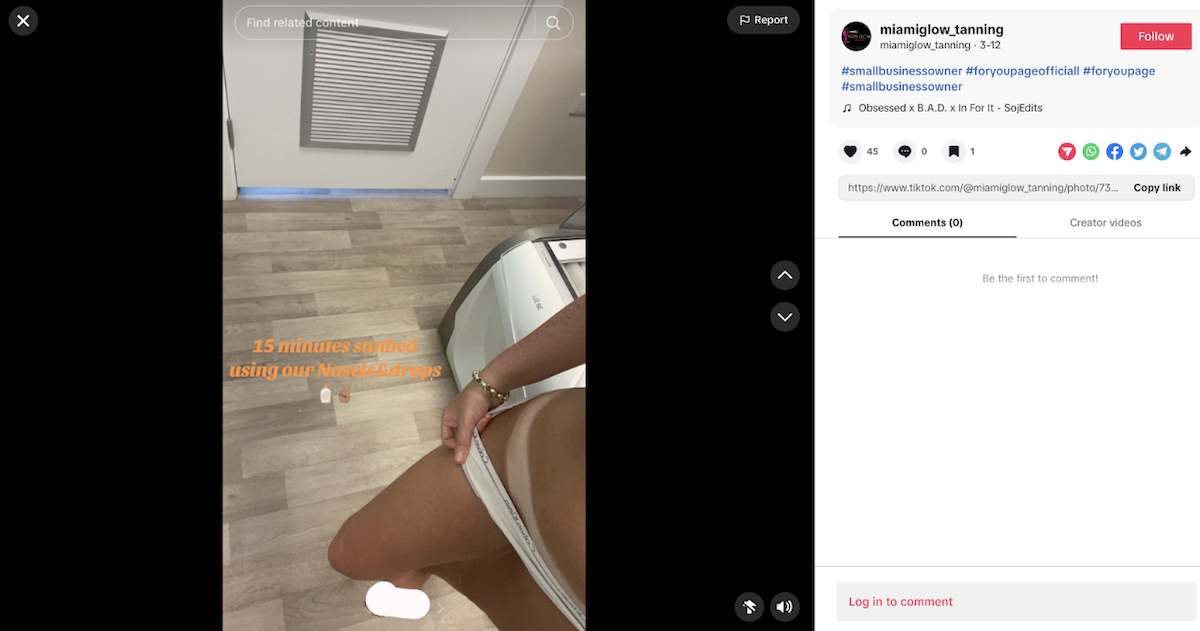
A social media influencer shows off her tan while marketing a melanotan nasal spray.
How Marketers of Melanotan II Attempt to Fly Under the Radar
Despite its notable health risks, melanotan’s popularity has not waned. Its regulatory status has not halted demand either. TikTok has taken measures to mitigate the dangerous trend by removing videos promoting or selling Melanotan II and nasal tanning spray, as well as banning hashtags including #nasaltanningspray, #melanotan, and #melanotan2. However, as LegitScript has previously noted with the unapproved drug Apetamin, many sellers have simply adjusted their marketing in order to avoid scrutiny.
Between 2017-2021, the melanotan-related merchants encountered by LegitScript were almost exclusively reported with the BRAM and VIRP actions, indicating that these merchants were blatantly marketing the banned product. In recent years, however, LegitScript analysts have increasingly used the Suspected Transaction Laundering action in order to note the risk presented by bad actors who avoid the term “melanotan” while marketing generically packaged nasal tanning sprays that bear striking resemblance to the drug. Analysts have learned to look for the following common characteristics that indicate a merchant’s tanning product may be repackaged Melanotan II:
- The products may appear in generic plastic containers without apparent safety seals.
- The products may display overly simplistic and unprofessional-looking labels. They may be self-branded with the merchant’s logo, or display no label at all.
- The products typically lack an ingredients list and often have a sparse product description.
- The marketing may describe the application and purpose commonly associated with Melanotan II (e.g. “nasal” language, references to tanning “strengths”).
- The marketing may include images of individuals with deeply tanned skin, stark tan lines, and before-and-after skin comparisons.
- The marketing may refer to the products as “MT2” or simply “2,” common abbreviations for Melanotan II.
- The product packaging may mimic the form factors of those known to contain Melanotan II (e.g. small bottles with nasal spray caps).
LegitScript Analysts Continuously Adapt to Detect Risk
As regulatory and media scrutiny has increased, many merchants have continued to sell illegal products, attempting to fade into the shadows and avoid consequences. However, their detection remains imperative to the well-being of consumers and the safety of the internet and payment ecosystems. LegitScript has paid close attention to the shifting online presence of Melanotan II and nasal tanning sprays, learning common marketing strategies and allowing analysts to identify nuanced risk factors that may be missed by measures such as social media tag bans. LegitScript analysts continue to vigilantly monitor and report any risk associated with Melanotan II.




