The Federal Trade Commission (FTC) and US Food and Drug Administration (FDA) announced Monday seven warning letters they issued jointly to business owners making potentially problematic claims that their products can treat or prevent the new coronavirus. These letters appear to reflect aggressive action by the federal agencies to stop businesses capitalizing on COVID-19 fears by making unsupported claims about their products' abilities to treat the disease.
Products cited in the letters include teas, essential oils, tinctures, and colloidal silver, a product made of microscopic silver typically suspended in demineralized water. The FDA has stated there are no approved vaccines, drugs, or investigational products currently available to treat or prevent COVID-19.
The warning letters call out unsupported claims made on company websites, social media accounts, and videos. It's important to note that merchants can be held responsible for claims made on any content they control, not only information on their official company websites.
For example, the warning letter for Pure Vital Silver, which markets colloidal silver products, referenced numerous unsupported claims regarding COVID-19 treatment on its associated Facebook page. At the time of our review, most of these claims were still live.
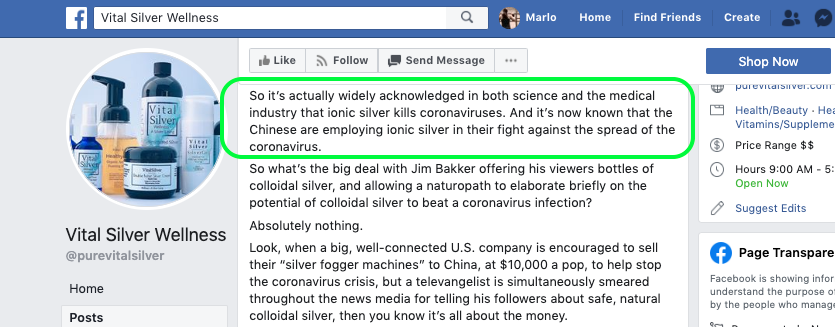
One of four posts on Pure Vital Silver's Facebook page cited in a warning letter
An essential oils merchant, Herbal Amy, Inc., was cited for selling a bundle called Coronavirus Protocol, which includes four products marketed for, "[i]mmune system, cellular protection, cytokine interruption tincture formulation, supportive for core tincture activity," and more.
The warning letter called out the following claim on the Coronavirus Protocol bundle webpage: "Corona virus treatment. ... [T]his is a rather extensive protocol because the particular corona virus that is now spreading world wide is exceptionally potent in its impacts. All the herbs are specific in one way or another for this virus. A number of the herbs are strongly antiviral for corona viruses ..... The formulations are preventative as well as specific for acute infections …"
Although the cited claim appears to have been removed, the webpage is still active.
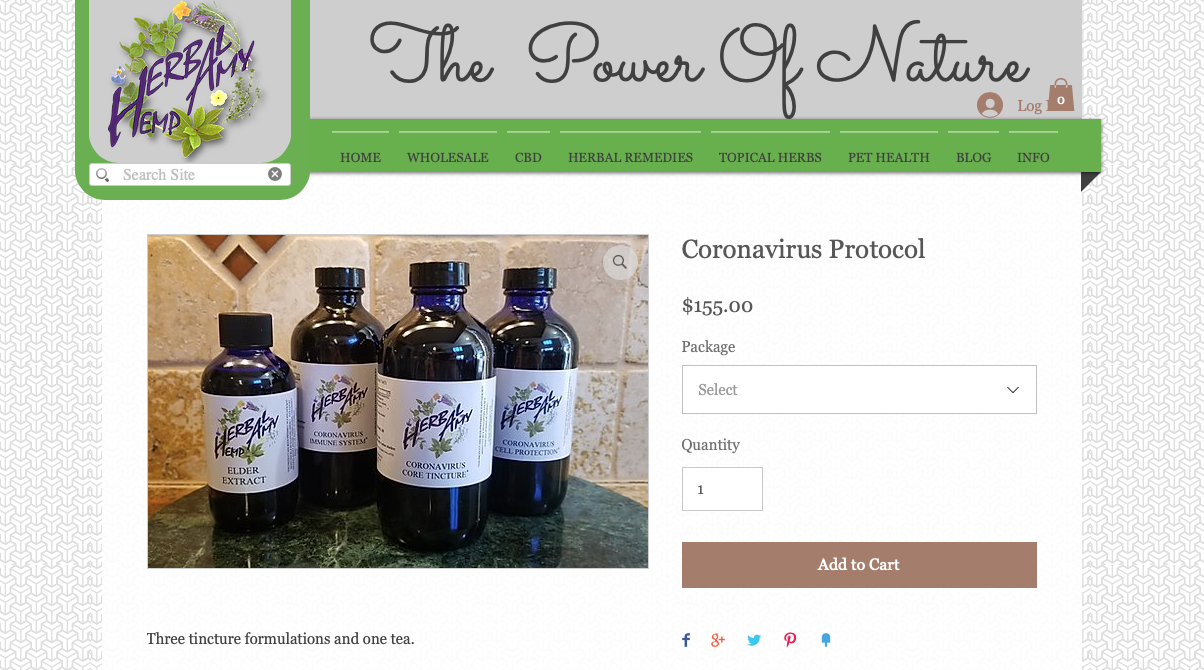
Essential oils marketed as "Coronavirus Protocol"
One of the warning letters was sent to televangelist and convicted fraudster Jim Bakker, whose website for The Jim Bakker Show markets Silver Solution and Silver Gel, colloidal silver products. In addition to claims made on the website, the letter called out assertions made in an episode of The Jim Bakker Show that has since been removed from the website. In the episode, guests make a variety of claims, including:
- "Silver Solution has been proven ... to kill every pathogen it has ever been tested on ... and it can kill any of these known viruses … ."
- "So the virus, like the coronavirus that we're talking about ... affects the lung tissue so what you can do ... put it straight ... in a nebulizer which then creates a steam and you breathe it in and it will go directly into your lungs where that virus is and any other infection."

Homepage of The Jim Bakker Show website marketing colloidal silver
Clips of the episode are still available on other media outlets.
As long as the COVID-19 epidemic persists, payment service providers may want to carefully scrutinize both new and existing merchants for unsupported claims around the new coronavirus. According to the press release, FTC and FDA are actively monitoring company websites, social media, online marketplaces, and incoming complaints for these types of claims. For more information on spotting scammers trying to cash in on the outbreak, see the FTC's coronavirus blog post.