The US Food and Drug Administration (FDA) recently released a consumer update warning against fraudulent flu products on the market in 2022-2023 flu season. LegitScript has identified websites that appear to be marketing homeopathic products and dietary supplements claiming to treat or cure seasonal flu. Keep reading to understand the FDA’s warning and some problematic claims made by these products.
Some Fraudulent Flu Products Use Problematic Marketing — Prompting FDA Warning Letters
According to the FDA, if “a product is intended to treat, diagnose, cure, or prevent diseases, it is a drug, even if it is labeled as a dietary supplement.” The FDA actively pursues companies misbranding supplements and over-the-counter products.
Merchants making false drug claims may face a warning letter. In an October 2022 letter issued to Rosebud's Ranch and Garden, LLC, the administration pointed to impermissible claims on the merchant’s website and social media accounts about their tea products’ ability to treat the flu and other diseases.
Some of these claims, according to the warning letter, included:
- “Eases the discomfort of a cold or flu”
- “Eases Muscular Pain”
- “Cancer Preventer”
- “Blood Sugar Balancer”
- “lowering bad cholesterol”
The letter stated that the “claims on your websites establish the products are drugs under section 201(g)(1)(B) of the Federal Food, Drug, and Cosmetic Act (the Act) [21 U.S.C. 321(g)(1)(B)] because they are intended for use in the cure, mitigation, treatment, or prevention of disease.”
In the course of our monitoring work, LegitScript has identified websites marketing dietary supplements, vitamins, and herbs with claims they increase immunity and reduce the symptoms of upper respiratory illnesses like the flu.
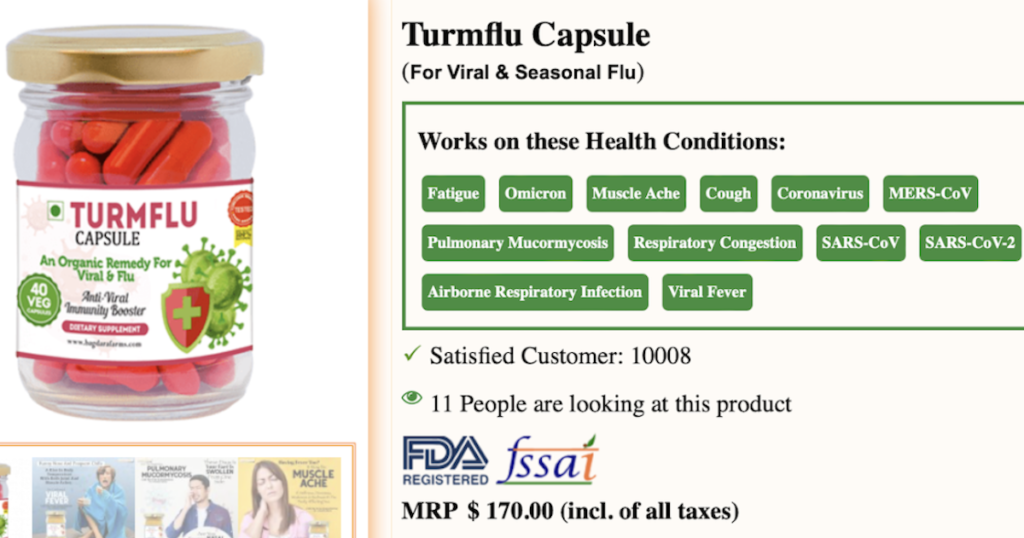
A website known to LegitScript markets an “Anti-Viral Immunity Booster” dietary supplement with language that claims it treats respiratory infections, viral fever, seasonal flu, COVID-19, and more.
Products Marketed Problematically May Include These Types of Claims
The FDA has warned consumers to look out for potentially fraudulent flu and antiviral products being sold without a prescription. Such products may claim to:
- Reduce the severity and length of flu or other viral infections
- Boost your immunity naturally without a flu vaccine
- Act as a safe and effective alternative to the flu vaccine
- Prevent catching the flu or viral infections
- Be an effective treatment for flu or viral infections
- Provide faster recovery from the flu or viral infections
- Support your body’s natural immune defenses to fight off flu or other viruses
- Want to Learn More About How To Correctly Advertise Supplements Online?
Are you a supplement seller, or do you process payments for supplement merchants? Our comprehensive guide The Dos and Don’ts of Internet Supplement Ads sheds light on common compliance issues supplement merchants face every day. Topics covered include everything from sponsored content and endorsements to user experience and popups. Click the image or button below to download your free guide today.





